Pathophysiology of Diabetes Mellitus
Diabetes = Hyperglycemia, which is defined
as:
--fasting
plasma glucose above 126 mg/dl
--oral
glucose tolerance test (OGTT) above 200 mg/dl
Pre-Diabetes is defined as:
--impaired
fasting glucose (IFG) of 100-125 mg/dl
--impaired
glucose tolerance (IGT) of 140-199 mg/dl
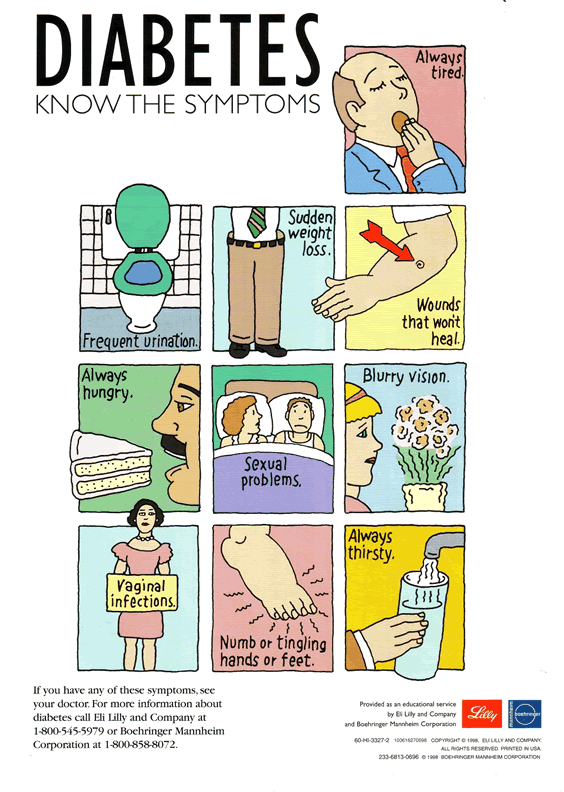
Acute
Complications of Uncontrolled Diabetes (all
directly caused by hyperglycemia)
--Polydypsia due
to plasma glucose hyperosmolarity
--Polyuria due to excess fluid intake and
glucose-induced osmotic diuresis
--Weight loss due to calories lost as
glucosuria, leaving a negative calorie balance
--Polyphagia due to glucosuria and negative
calorie balance
--Poor wound healing, gingivitis, blurred
vision
Chronic Complications of Uncontrolled Diabetes
Chronic complications may be due to mitochondrial
superoxide overproduction in response to hyperglycemia.
Macrovascular Atherosclerosis: diabetics have a high
incidence of coronary, cerebral, and peripheral
artery diseases. Caused by dyslipidemias including elevated LDL and
triglycerides, low HDL, and reduced
fibrinolytic activity. Management
includes foot care.
Microvascular Diseases
of Diabetes
1. Diabetic Retinopathy has many manifestations,
including microaneurysms, microhemorrhages, proliferative
vessel changes, and vitreous bleeds (cause blindness). Diabetic retinopathy is caused by basement membrane deterioration and
ischemia.
2. Nephropathy progresses from microalbuminuria to
proteinuria to uremia to ESRD.
Nephropathy is caused by
hyperfiltration, increased glomerular pressure, and BM thickening.
3. Neuropathy
can be peripheral (symmetrical stocking-glove numbness/tingling), autonomic (erectile dysfuntion, enteropathy,
gastroparesis, orthostatic hypotension), or mononeuropathic
(single nerve pain/palsy). Peripheral
and autonomic neuropathies are caused
by metabolic schwann cell defects and
axonal degeneration.
Mononeuropathies are
caused by ischemia, and resolve on
their own within a year.
The
Diabetic Foot
This is a combination of Peripheral
Neuropathy (leads to undetected trauma, blisters, ulcers) and Peripheral Vascular Disease (impairs
healing, allows infection).
Endstage Complications of Uncontrolled Diabetes
1. Diabetic
Ketoacidosis results from complete
lack of insulin and reliance on fatty acids for energy. There is
unrestrained lipolysis and ketone synthesis, causing acidosis and ketonemia. Patients will show Kussmaul respiration. This
is a medical emergency.
2. Non-Ketotic
Hyperosmolarity is an extreme hyperglycemia without acidosis. It is caused by insufficient insulin
resulting in poor glucose uptake and increased hepatic glucose output. There
is just enough insulin to suppress ketone synthesis. The extreme hyperglycemia leads to osmotic diuresis and vascular
collapse.
Diabetic
Control
1.
Self-monitoring of blood glucose
2. Hemoglobin A1c (“glycohemoglobin”) monitoring
measures glycemia over the last 2-3 months.
Hemoglobin
A1c < 7 is good. Hemoglobin A1c >
9 is bad.
Type 1 Diabetes Mellitus
Characterized
by Beta cell deficiency, leading to complete
insulin deficiency. This leads to severe metabolic
lability and ketoacidosis. Metabolic
lability is hard to treat.
DM type 1 is
almost always an autoimmune disease
caused by anti-islet, anti-GAD, or anti-insulin
antibodies. These cause lymphocytic inflitration and
destruction of the pancreas islets. Although the destruction is gradual, the clinical onset is usually acute.
DM type 1 is
associated with other autoimmune conditions including vitiligo and
hypothyroidism.
DM type 1
usually occurs in people <35 yo who are of thin-to-normal body weight. It is much less
common than DM type 2, and shows some HLA associations. Family
history is rare.
DM type 1
always requires insulin therapy, and will not respond to insulin-stimulating
oral drugs.
Remember that metabolic lability and
glucose swings are a hallmark of DM type 1.
*End-stage DM type 1 is ketoacidosis.
Type 2 Diabetes Mellitus
Characterized by insulin resistance and relative,
not complete insulin deficiency.
DM type 2 is caused by Beta cell deficiency coupled with peripheral insulin resistance.
--Peripheral
insulin resistance is the hallmark of DM type 2. Although insulin levels may be high, this does not
cause hypoglycemia. This is due to
receptor density and post-receptor
changes in response to chronic hyperinsulinemia.
--Obesity
is the main cause of insulin resistance.
--Beta
cell deficiency occurs over years as islets fail from insulin
overproduction. Insulin levels become inadequate
to overcome the peripheral resistance.
DM type 2
develops when insulin production can no
longer overcome peripheral insulin resistance.
Patients become unresponsive to
insulin-stimulating oral drugs, and ultimately require insulin injections. But
because there is always some residual insulin secretion, there is little
metabolic lability and glucose levels tend to stay stable.
DM type 2 usually occurs in people >35
yo who are obese. Family
history is very common, and twin
concordance is extremely high.
*End-stage DM type 2 is Non-Ketotic Hyperosmolarity.
|
|
Type 1 Diabetes
|
Type 2 Diabetes
|
|
Etiology
|
Autoimmune
|
Peripheral
resistance
|
|
Formerly known as
|
IDDM
|
NIDDM
or “adult onset” diabetes
|
|
Age of onset
|
Younger
|
Older
|
|
Obesity
|
Rare
|
Common
|
|
Family History/Twin concordance
|
Rare
|
Common
|
|
HLA association
|
Yes
|
No
|
|
Ketosis
|
Yes
|
No
|
|
Insulin resistance
|
No
|
Yes
|
|
Endogenous insulin
|
No
|
Yes
|
|
Respond to Oral Agents
|
No
|
Yes
|
|
Metabolic lability
|
Labile
|
Not
labile
|
(Raymond removed image of natural history
of type 2 diabetes: glucose levels, insulin resistance and secretion.)
Other
causes of hyperglycemia
1. Gestational diabetes is caused by excess counter-insulin hormones of pregnancy,
which lead to insulin resistance. The resulting maternal hyperglycemia is
transmitted to the fetus, causing fetal
pancreatic hypertrophy. The baby will be
large and fat, with neonatal complications.
2.
Defective insulin receptors or post-receptor signal transduction.
3.
“Glucose toxicity” in which hyperglycemia itself causes more insulin
resistance.
This
is a feedforward cycle that leads to ketoacidosis and severe hyperglycemia.
Carbohydrate Metabolism and Hypoglycemia
Increased blood glucose stimulates insulin
secretion.
High insulin will promote glucose uptake, glycolysis,
and glycogenesis, as well as uptake and synthesis
of amino acids, proteins, and fat.
Low insulin will promote gluconeogenesis,
glycogenolysis, lipolysis, and proteolysis.
|
|
Liver
|
Adipose Tissue
|
Muscle
|
|
High
insulin
|
Glycolysis
Glycogenesis
|
Triglyceride synthesis
|
Amino acid uptake
Protein synthesis
|
|
Low
insulin
|
Gluconeogenesis
Glycogenolysis
|
Lipolysis
|
Proteolysis
|
Normal
Responses to Eating and Fasting
1. CHO
fed state: increased insulin
secretion, causing glycolysis, glycogen storage, fatty acid synthesis/storage, and protein synthesis.
2. Overnight
fast: low insulin and high glucagon
cause glycogen breakdown, hepatic gluconeogenesis,
and lipolysis.
3. Prolonged
fast: extremely low insulin and low
glucagon cause lipolysis to take over.
Lipids are the main fuel
source. Gluconeogenesis is minimized, as
it causes nitrogen wasting, ammonia
build-up, and loss of muscle mass.
Counter-Regulatory
Hormones
The following hormones raise blood glucose and oppose insulin:
--Glucagon
--Epinephrine/Norepinephrine
--Cortisol
--Growth
Hormone
These are “fight or flight” hormones that
raise glucose to fuel muscles. They
cause anxiety, alertness,
apprehension, sweating, and tachycardia.
These hormones are released due to stress.
Therefore, stress worsens diabetic control, raises blood glucose, and generally worsens diabetes.
Hypoglycemia
Hypoglycemia is defined by “Whipple’s Triad”:
1. Documented low plasma glucose
2. Symptoms of hypoglycemia
3. Response to administered carbohydrate
There are two classes of hypoglycemic
symptoms:
1. Adrenergic
symptoms due to catecholamine release and counter-regulatory hormones. These
symptoms include tremor, blurred vision, weakness, palpitations, anxiety, emotional
lability, headache. Adrenergic symptoms
mildly dangerous.
2.
Neuroglucopenic symptoms due
to inadequate glucose to the brain.
These symptoms include
confusion, slurred speech, siezures, somnolence, and coma. Very dangerous.
Reactive
versus Fasting Hypoglycemia
It is not normal to become symptomatically
hypoglycemic, even during a prolonged fast!
Reactive/postprandial
Hypoglycemia occurs a few hours after a meal. It is common, and not associated with disease. May
be caused by overly rapid gastric emptying (“alimentary hypoglycemia”).
Fasting
Hypoglycemia occurs post-absorptively (>10 hours
after a meal). It is abnormal, and is usually
caused by excess insulin. This may
be exogenous insulin (over-injection during diabetes
therapy) or endogenous insulin (insulinoma). Insulinomas may be detected by putting patients on a prolonged fast
and looking for persistently high insulin levels or symptoms of hypoglycemia.
Other causes of fasting hypoglycemia
include defective gluconeogenesis
(liver failure, alcoholic hypoglycemia)
and counter-regulatory hormone deficiency
(adrenal insufficiency).

I started on COPD Herbal treatment from Ultimate Health Home, the treatment worked incredibly for my lungs condition. I used the herbal treatment for almost 4 months, it reversed my COPD. My severe shortness of breath, dry cough, chest tightness gradually disappeared. Reach Ultimate Health Home via their email at ultimatehealthhome@gmail.com . I can breath much better and It feels comfortable!
ReplyDeleteAfter years of battling COPD with little to no lasting relief, I turned to the herbal treatment from NaturePath Herbal Clinic—and it’s one of the best decisions I’ve made.
ReplyDeleteWithin just four months, I experienced noticeable improvements: easier breathing, reduced coughing, and a significant decrease in chest tightness. I'm now able to walk longer distances, sleep more soundly, and enjoy daily activities without constant fatigue or shortness of breath.
This treatment has genuinely transformed my quality of life. If you're seeking a natural and effective solution for COPD, I wholeheartedly recommend NaturePath Herbal Clinic.
Learn more: www.naturepathherbalclinic.com